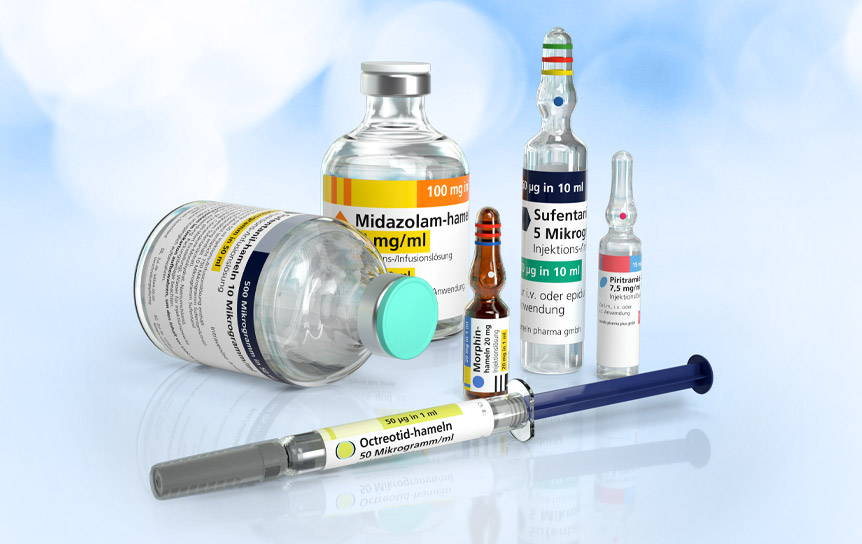
 Where quick help is needed or even vital, medical professionals can be under stress and time pressure: every patient needs specific therapy and care. The preparation of individual injections and infusions can be prone to errors. With a keen focus on patient safety, hameln has developed a range of Ready-to-Use products which help minimise risks when preparing and administering injectable medicines.
Where quick help is needed or even vital, medical professionals can be under stress and time pressure: every patient needs specific therapy and care. The preparation of individual injections and infusions can be prone to errors. With a keen focus on patient safety, hameln has developed a range of Ready-to-Use products which help minimise risks when preparing and administering injectable medicines.
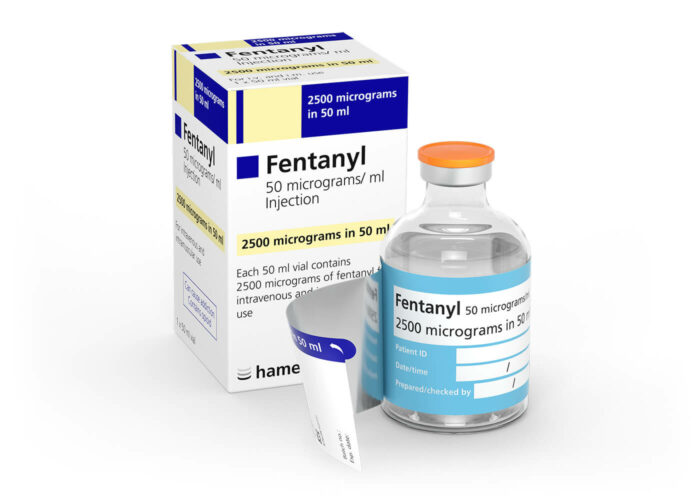
Our Ready-to-Use solutions with Integrated Syringe Label® offer decisive advantages:
– they reduce preparation errors in injectable medicines
– they reduce the risk of contamination
– they allow for more time spent caring for the patient
The design of the syringe label follows national and international standards, such as the recommendations of the Intensive Care Society as well as ISO colours.
Four simple steps that will increase patient safety and save time
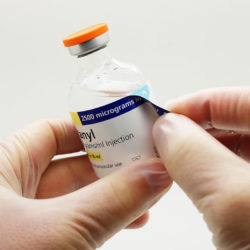

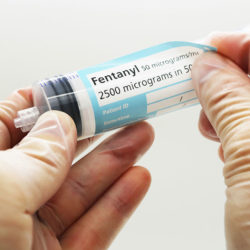
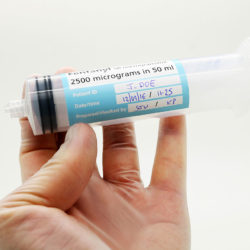
Labelling medication made quick and easy:
Peel back the outer label to reveal the syringe label that is supplied with the vial before applying to a syringe. Drug preparation is made quicker and easier, replacing the need for handwritten syringe labels and ensuring the contents of prepared syringes will be easier to identify during administration as well as during dose preparation.